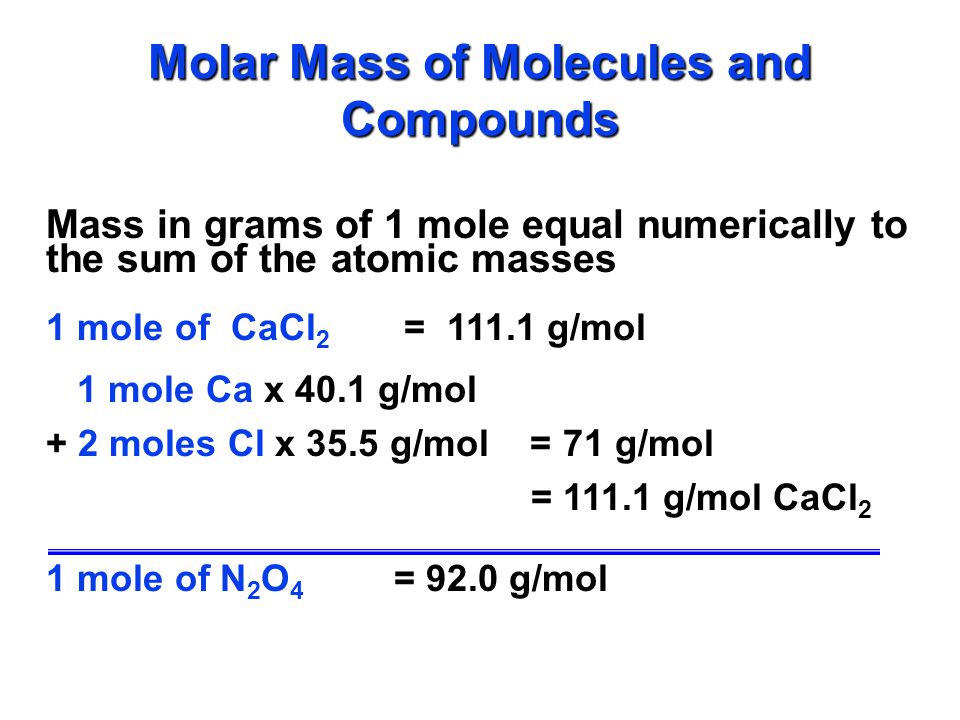
The Mole Standards 1 dozen = 1 gross = 1 ream = 1 mole = x There are exactly 12 grams of carbon-12 in one mole of carbon ppt download

The Mole What's a mole? In chemistry, a mole is a counting unit. What does that mean? –1–1 dozen = 12 units(ie eggs,donuts) –1–1 mole = 6.02x10 23 particles. - ppt download

The Mole & Chemical Quantities. The Mole Mole-the number of particles equal to the number of atoms in exactly 12.0 grams of carbon mol = 6.02 x. - ppt download


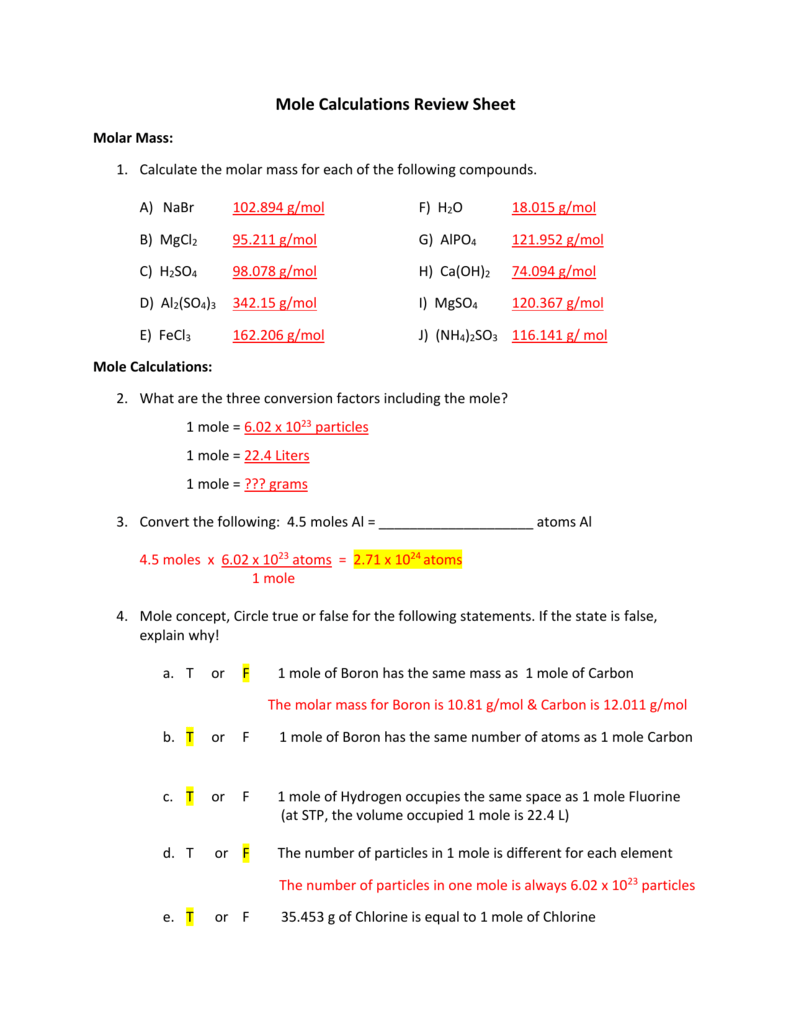




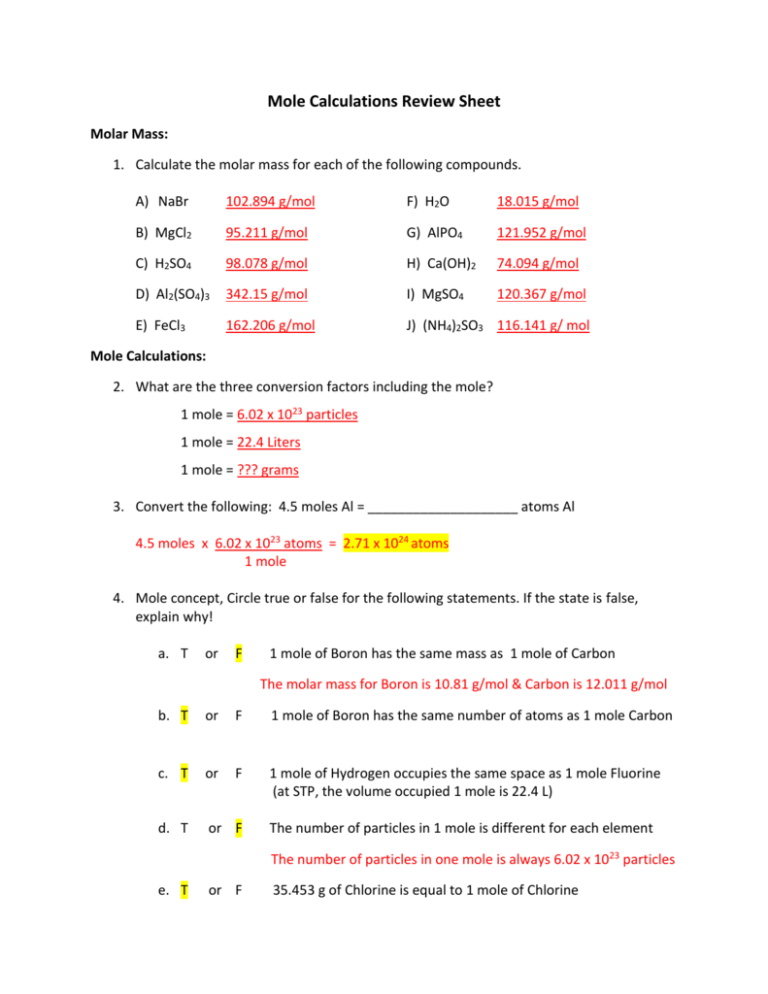
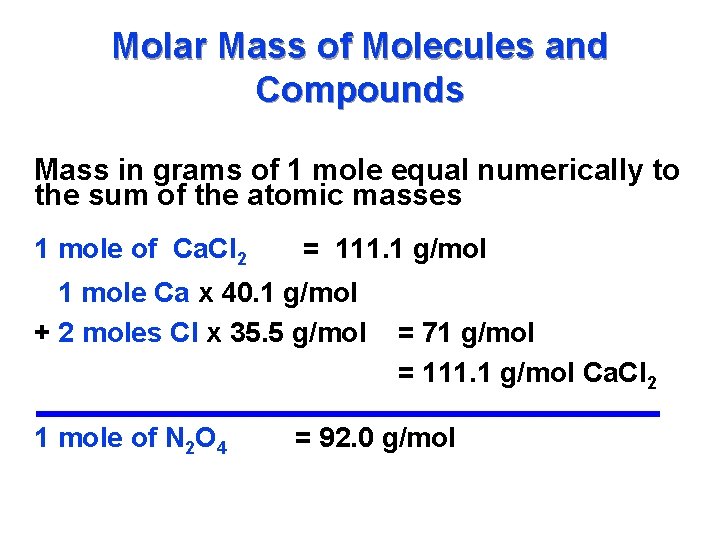






/what-is-a-mole-and-why-are-moles-used-602108-FINAL-CS-01-5b7583f6c9e77c00251d4d68.png)